SUMMARY
The methyl esters of 3,5-dibromo-N-phenylanthranilic acids have been synthesized. The structure of the compounds was confirmed by elemental analysis, IR- and NMR-spectroscopy, and individuality by chromatography in a thin layer of sorbent. The results of pharmacological studies suggest that esterification of the carboxyl group of 3,5- dibromo-N-phenylanthranilic acids leads to the decrease in anti-inflammatory, analgesic and diuretic activity, and leads to the increase of toxicity compared to the initial acids.
Key words: synthesis, N-phenylanthranilic acid, methyl esters, pharmacological properties.
Introduction. Derivatives of N- phenylanthranilic acids are used in medicine as a nonsteroidal antiinflammatory drugs. Some of them have proven to be most severe for nonspecific actions, and those that do not cause a degradation of glycosaminoglycans and kolagen of joints [1,7]. Today, derivatives of N- phenylanthranilic acids investigated as potential anti-cancer drugs, as well as those which could be applied in Alzheimer's disease and cardiovascular disease, that is very relevant and necessary, given the increase in morbidity data nosological forms [2-6, 8].
Given the above, the aim of our work was to obtain a number of biologically active compounds - methyl esters of 3,5-dibromo-N-phenylanthranilic acids and study of their pharmacological properties.
Substituted 3,5-dibromo-N-phenylanthranilic acids have been obtained by the Ullmann reaction by the interaction of 3,5 dybromo-2-chlorobenzoic acid with arylamines (method 1) and by arylation of 3,5-dibromoanthranilic acid by halogenbenzenes derivatives (method 2) in the media of n-amyl alcohol (way 1A, 2A), ) in the media of
dimethylformamide (way 1B, 2B), without solvent (way 1C, 2C) in the presence of copper or copper (II) oxide [6,8]. Also as a counter synthesis of 3,5-dibromo-N-phenylanthranilic acids condensation of N-acetyl-3,5-dybromoanthranilic acid with substituted halogenbenzene followed by hydrolysis of N-acyl derivatives has been used (method 3).
In order to increase the solubility of copper ions in aprotic low-polar phase to accelerate the arylation reaction various solubilizers (Tween-80, sodium salts of stearic acid, oleic acid) have been used, which have been added to the reaction mixture in an amount of 2-5 weight percent. Use of sodium oleate as a phase transfer catalyst allows to faster the arylation reaction in 1,7-3,5 times [9].
The synthesis of methyl esters of 3,5-dibromo-N-phenylanthranilic acids has been carried out by Fischer esterification in the media of absolute methanol in the presence of concentrated sulfuric acid (Table 1, Scheme 1):
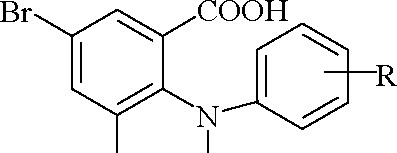
Br H
CHOH
(k. H2SO4)
І. ІІ. (2.1-2.11)
Scheme 1
The structure and identity of 3,5-dibromo-N-phenylanthranilic acids have been confirmed by elemental analysis, IR- and NMR-spectroscopy, chromatographic analysis and qualitative reactions.
In the NMR-spectra of esters signals of aromatic protons in 6,61-8,37 ppm have been identified. Secondary amino group’s proton signal appears as a broad singlet in the region of 8,32-9,38 ppm. Characteristic signal of methyl esters is a signal of OCH3 - group, which is registered in the 3,57-3,75 ppm (Table 2).
IR-spectra of the methyl esters of 3,5-dibromo-N-phenylanthranilic acids are characterized by a number of intense bands, which correspond to the main structural fragments of molecules of synthesized substances. In the area of 1704-
1670 cm -1 an intense band corresponding to stretching vibrations ester carbonyl group has been interpreted (vC - O). In been detected, respectively. The first band refers to the stretching vibration of C-O-C - group, to which the main
спирт.
і С-О ) have
the areas of 1250-1270 cm -1 and 1090-1050 cm -1 the stretching vibration bands of C-O-C (
ки сл .
vC - O
contribution is made by the fluctuations of “acidic” fragment of the molecule, the main contribution to the second band oscillations is made by the “alcoholic” fragment of the molecule. Stretching vibrations of the C-Br - bond with a band of medium intensity at 628-532 cm -1 (VC - Br). Symmetric and asymmetric vibrations of nitrogroup in the spectrogram yaa ya
(6-8) appear in the areas1525-1516 cm -1 ( NO2 ) and 1325-1306 cm -1 ( NO2 ) (Table 2).
An anti-inflammatory, analgesic, diuretic and bacteriostatic properties of synthesized methyl esters of 3,5- dibromo-N-phenylanthranilic acids have been studied.
Studies on anti-inflammatory and analgesic activities have revealed that methyl esters did not exceed the reference drug, and some of them never showed these activities (Table 3), the original acids’ pharmacological activities had same or even higher activities than reference drugs (Table 4).
Methyl esters exhibited bacteriostatic activity in relation to Staphylococcus aureus, Bacillus subtilis, Echerichia coli and Pseudomonas aureginosa.
The esterification of the carboxylgroup of 3,5-dibromo-N-phenylanthranilic acids results in toxicity increase (Table 3 and 4).
Table 1 - Methyl esters of 3,5-dibromo-N-phenylanthranilic acids
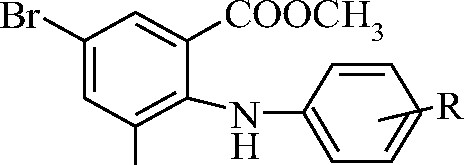
Br
|
Co mpo -und |
R |
Yeild, % |
Melting point оС1 |
Quantified, % |
Gross formula |
Calculated, % |
Rf2 |
|||
|
N |
C |
N |
C |
1 |
2 |
|||||
|
2.1 |
H |
88 |
162-164 |
3,7 1 |
43,80 |
С14Н11 Br2NO2 |
3,63 |
43,66 |
0,3 9 |
0,5 0 |
|
2.2 |
2’-CH3 |
84 |
187-189 |
3,4 5 |
45,03 |
C15H13 Br2NO2 |
3,50 |
45,14 |
0,3 8 |
0,4 9 |
|
2.3 |
4’-CH3 |
87 |
154-157 |
3,5 4 |
45,21 |
C15H13 Br2NO2 |
3,50 |
45,14 |
0,4 0 |
0,4 7 |
|
2.4 |
3’,4’- CH3 |
87 |
204-207 |
3,4 3 |
46,58 |
C16H15 Br2NO2 |
3,38 |
46,51 |
0,3 5 |
0,4 5 |
|
2.5 |
4’- OCH3 |
82 |
195-197 |
3,3 2 |
43,44 |
C15H13 Br2NO3 |
3,37 |
43,40 |
0,3 7 |
0,4 4 |
|
2.6 |
4’- OC2H5 |
85 |
109-111 |
3,3 4 |
44,75 |
C16H15Br2 NO3 |
3,26 |
44,78 |
0,6 2 |
- |
|
2.7 |
2’-NO2 |
80 |
120-122 |
6,5 9 |
39,14 |
C14H10Br2 N2O4 |
6,51 |
39,09 |
0,5 7 |
- |
|
2.8 |
3’-NO2 |
74 |
102-103 |
6,5 8 |
39,01 |
C14H10Br2 N2O4 |
6,51 |
39,09 |
0,5 8 |
- |
|
2.9 |
4’-NO2 |
80 |
88-90 |
6,5 5 |
39,03 |
C14H10Br2 N2O4 |
6,51 |
39,09 |
0,5 5 |
- |
|
2.10 |
4’-Br |
78 |
155-158 |
3,4 9 |
40,12 |
C14H10Br3 NO2 |
3,33 |
40,08 |
0,3 0 |
0,3 8 |
|
2.11 |
4’-Cl |
79 |
159-161 |
2,9 8 |
36,22 |
C14H10Br2 ClNO2 |
3,01 |
36,24 |
0,2 8 |
0,3 5 |
Note: 1 Crystallized from aqueous methanol;
2Rf values are given in solvent systems: 1. Methanol-hexane (1:3).
2.ethyl acetate- methanol -ammonia (8,5:1:0,5)
Table 2. - IR- and NMR-data of methyl esters of 3,5-dibromo-N-phenylanthranilic acids
|
R |
IR-spectra |
NMR -spectra |
||||||||||||
|
Absorption freq |
uency |
Chemical shift, 5, m.p. |
||||||||||||
|
Vnh |
acid. vC=O |
vC - О |
acacid. vC - О |
clcohi vC - О |
'l as v no 2 s |
^NH |
vC - Br |
vC - P |
NH 1 (1H, s.br. ) |
-CH3 |
- OCH2 - |
COOC H3 (3H,c) |
Ar-H |
|
|
Н |
332 2 |
1658 |
1702 |
1279 |
1082 |
- |
1588 |
584 |
160 5 |
9,32 |
- |
- |
3,61 |
6,917,93 (7H,m ) |
|
2’- CH3 |
333 0 |
1662 |
1699 |
1274 |
1085 |
- |
1575 |
605 |
160 0 |
9,19 |
2,07 (3H,s) |
- |
3,62 |
6,937,91 (6H,m ) |
|
4’- CH3 |
333 4 |
1664 |
1700 |
1272 |
1085 |
- |
1576 |
618 |
159 8 |
- |
- |
- |
- |
- |
|
3’,4’ CH3 |
334 2 |
1670 |
1695 |
1280 |
1087 |
- |
1574 |
620 |
160 5 |
9,05 |
2,19 (3H,s) 2,25 (3H,s) |
- |
3,62 |
6,707,93 (5H,m ) |
|
4’- OC H3 |
333 8 |
1672 |
1704 |
1285 |
1090 |
- |
1578 |
624 |
160 7 |
- |
- |
- |
- |
- |
|
4’- OC2 H5 |
332 5 |
1648 |
1688 |
1276 |
1057 |
1516 1306 |
1570 |
532 |
159 8 |
8,32 |
1,31 (3H,s) |
3,90 (2H,s) |
3,57 |
6,828,10 (6H,m ) |
|
2’- NO2 |
331 0 |
1626 |
1675 |
1270 |
1052 |
1525 1312 |
1574 |
540 |
160 0 |
9,01 |
- |
- |
3,75 |
6,618,37 (6H,m ) |
|
3’- NO2 |
332 0 |
1624 |
1670 |
1272 |
1050 |
1520 1310 |
1578 |
545 |
150 0 |
- |
- |
- |
- |
- |
|
4’- NO2 |
329 5 |
1625 |
1685 |
1272 |
1056 |
- |
1572 |
540 |
159 6 |
- |
- |
- |
- |
- |
|
4’- Br |
334 8 |
1650 |
1695 |
1285 |
1072 |
- |
1576 |
622 |
159 8 |
9,40 |
- |
- |
3,61 |
6,907,94 (6H,m ) |
|
Д’- Cl |
335 8 |
1648 |
1698 |
1290 |
1082 |
- |
1578 |
628 |
160 0 |
9,38 |
- |
- |
3,60 |
6,747,88 6H,m) |
Table 3. - pharmacological activities of methyl esters of 3,5-dibromo-N-phenylanthranilic acids
|
Compound |
R |
Anti- inflamma tory, % in a dose of 10 mg/kg |
Analgesic, % in a dose of 20 mg/kg |
Diuretic, % in a dose of 50 mg/kg (control group 100%) |
Bacteriostatic, MIC, Lg/ml |
DL50, mg/kg intra gastri cally (on mice) |
|||
|
Staphylo coccus aureus, ATCC 25923 |
Bacteriu m subtilis, ATCC 6639 |
Echerich ia coli, ATCC 25922 |
Pseudomo nas aureginosa ATCC 97853 |
||||||
|
2.1 |
H |
6,8 |
12,4 |
122 |
125 |
250 |
125 |
250 |
- |
|
2.2 |
2’-CH3 |
12,7 |
19,3 |
78 |
125 |
250 |
125 |
250 |
- |
|
2.3 |
3’-CH3 |
0 |
0 |
134 |
250 |
500 |
125 |
250 |
- |
|
2.4 |
3’,4’- CH3 |
31,2 |
34,8 |
95 |
250 |
500 |
62,5 |
62,5 |
>1500 |
|
2.5 |
4’- OCH3 |
24,5 |
0 |
75 |
250 |
500 |
125 |
250 |
- |
|
2.6 |
4’- OC2H5 |
0 |
36,1 |
108,4 |
62,5 |
62,5 |
62,5 |
250 |
>1200 |
|
2.7 |
2’-NO2 |
0 |
0 |
98,3 |
62,5 |
62,5 |
31,2 |
125 |
- |
|
2.8 |
3’-NO2 |
0 |
0 |
- |
31,2 |
62,5 |
31,2 |
125 |
- |
|
2.9 |
4’-NO2 |
0 |
0 |
- |
31,2 |
62,5 |
31,2 |
125 |
- |
|
2.10 |
4’-Br |
22,1 |
29,3 |
- |
125 |
250 |
125 |
250 |
- |
|
2.11 |
4’-Cl |
29,5 |
35,1 |
132 |
125 |
250 |
125 |
250 |
>1500 |
|
Diclofenac (ED50=8 mg/kg) |
37,5 |
- |
- |
- |
- |
- |
- |
360 |
|
|
Mefenamic acid in a dose of 100 mg/kg |
30 |
- |
- |
- |
- |
- |
- |
628 |
|
|
Metamizole sodium ED50=55 mg/kg |
- |
52,0 |
- |
- |
- |
- |
- |
1197 |
|
|
Hydrochlorothia zide in a dose of 50 mg/kg |
- |
- |
212 |
- |
- |
- |
- |
320 |
|
|
Ethacridine |
- |
- |
- |
31,2 |
15,6 |
31,2 |
62,5 |
- |
|
|
Phthalylsulfathia zole |
- |
- |
- |
7,8 |
7,8 |
250 |
- |
- |
|
Conclusions. A synthesis of methyl esters of 3,5-dibromo-N-phenylanthranilic acids has been carried out.
By means of physico-chemical methods - elemental analysis, IR and NMR spectroscopy, TLC the structure of compounds have been confirmed. Pharmacological studies of methyl esters of 3,5-dibromo-N-phenylanthranilic acids have been carried out. The esterification of the carboxylgroup of 3,5-dibromo-N-phenylanthranilic acids results in toxicity increase and in reduce of pharmacological activity.
Table 4. - pharmacological activities of 3,5-dibromo-N-phenylanthranilic acids
|
Com pound |
R |
Antiinflammatory, % in a dose of |
Analgesi c, % in a dose of 20 mg/kg |
Diuretic, % in a dose of 50 mg/kg |
Bacteriostatic, MIC, pg/ml |
DL50, mg/kg intra Gastri cally (on mice) |
||||
|
10 mg/kg |
20 mg/k g |
St. Aureus, ATCC 25923 |
Bacte rium subtilis, ATCC 6639 |
Ech. Coli, ATCC 25922 |
Pseud. Auregi nosa, ATCC 97853 |
|||||
|
2.1 |
Н |
- |
12,6 |
20,5 |
132,9 |
250 |
500 |
250 |
250 |
- |
|
2.2 |
2’-CH3 |
- |
14,2 |
22,7 |
180,2 |
250 |
250 |
250 |
500 |
- |
|
2.3 |
3’-CH3 |
- |
34,5 |
39,8 |
216,2 |
250 |
500 |
250 |
250 |
>3000 |
|
2.4 |
3’,4’- CH3 |
39,5 |
44,2 |
40,5 |
218,5 |
250 |
500 |
125 |
125 |
>3500 |
|
2.5 |
4’-OCH3 |
0 |
10,2 |
0 |
225 |
250 |
500 |
250 |
250 |
- |
|
2.6 |
4’- OC2H5 |
14,1 |
- |
0 |
185,6 |
62,5 |
125 |
125 |
125 |
>3000 |
|
2.7 |
2’-NO2 |
26,7 |
- |
9,5 |
120,5 |
62,5 |
31,2 |
62,5 |
125 |
>2500 |
|
2.8 |
3’-NO2 |
21,6 |
- |
- |
140,0 |
31,2 |
62,5 |
125 |
125 |
- |
|
2.9 |
4’-NO2 |
11,6 |
- |
- |
- |
31,2 |
62,5 |
125 |
125 |
- |
|
2.10 |
4’-Br |
25,1 |
35,4 |
39,2 |
284,5 |
250 |
250 |
125 |
125 |
>3500 |
|
2.11 |
4’-Cl |
32,5 |
40,8 |
30,4 |
290 |
250 |
250 |
125 |
125 |
>3500 |
LITERATURE
- Машковский М.Д. Лекарственные средства / Изд. 15-е, перераб., испр. и доп. - М.: Новая волна, 2005. - 1200 с.
- Tsutomu, Nakahara. Vascular Pharmacol / Nakahara Tsutomu, Mitani Akiko, Saito Maki et al // 2004. - Vol.41, Iss. 1. P. 21-25.
- Gwanyanya Asfree. Inhibition of the calcium-activated chloride current in cardiac ventricular myocytes by N-(p- amylcinnamoyl)anthranilic acid (ACA) / Asfree Gwanyanya, Regina Macianskiene, Virginie Bito, Karin R. Sipido et al. // Biochemical and Biophysical Research Communications. - 2010. Vol. 402, Issue 3. - P. 531-536.
- Lloyd J.The synthesis and structure-activity relationship of substituted N-phenyl anthranilic acid analogs as amyloid aggregation inhibitors / Lloyd J. Simons, Bradley W. Caprathe, Michael Callahan, James M. Graham et al. // Bioorganic & Medicinal Chemistry Letters. - 2009. Vol. 19, Issue 3. - P. 654-657.
- Darby Schmidta. Anthranilic acid replacements in a niacin receptor agonist / Darby Schmidta, Abigail Smentona, Subharekha Raghavana, Hong Shena et al. // Bioorganic & Medicinal Chemistry Letters. - 2010. Vol. 20, Issue 11. - P. 3426-3430.
- Varnavas Antonio. Anthranilic acid based CCK1 receptor antagonists: preliminary investigation on their second “touch point” / Antonio Varnavas, Lucia Lassiani, Valentina Valenta, Laura Mennuni et al. // European Journal of Medicinal Chemistry. - 2005. Vol. 40, Issue 6. - P. 563-581.
- Sharma Shalabh. Newer N-substituted anthranilic acid derivatives as potent anti-inflammatory agents / Shalabh Sharma, Virendra Kishor Srivastava and Ashok Kumar // European Journal of Medicinal Chemistry. - 2002. Vol. 37, Issue 8. - P. 689-697.
- Cocco Maria T. Synthesis of new N-(2-(trifluoromethyl)pyridin-4-yl)anthranilic acid derivatives and their evaluation as anticancer agents / Maria T. Cocco, Cenzo Congiu, Valentina Lilliu, Valentina Onnis // Bioorganic & Medicinal Chemistry Letters. - 2004. Vol. 14, Issue 23. - P. 5787-5791.