Summary: Multi-target drug discovery represents an innovative approach of medicinal chemistry to overcome the crisis in drug design. This vision is useful in the designing compounds with particular antimicrobial, antineoplastic, and anti-inflammatory activity, because it is advantageous if the drug have other supportive effects. Cinnamic acid derivatives are known to have a variety of biological effects and can be considered as a typical representative of the above-mentioned approach. This contribution focuses on the investigation of new ring-substituted cinnamanilides with estimated anti-microbial and antiinflammatory activity.
Keywords: anti-inflammatory agents, anti-invasive agents, cinnamic acid, multi-target agents
Introduction
Multi-target drug discovery represents an innovative approach of medicinal chemistry to overcoming a crisis in drug design, especially of anti-invasive drugs, reflected in the small number of newly approved drugs. This approach is based on the concepts of privileged scaffolds, polypharmacology, and multifactorial diseases [1–6]. This vision is very useful in the design of compounds with antimicrobial, antineoplastic, and anti-inflammatory activity, because it is advantageous when drugs have other supportive activities. From pharmacoeconomic and patients’ comfort points of view, it seems favorable to treat both a cause and a consequence (e.g., bacterial infection and inflammation) at one time by one active agent. Cinnamic acid ((2E)-3-phenylprop-2-enoic acid) [7] and its ring-substituted derivatives have been widely investigated with respect to their significant and varied biological effects, such as anti-inflammatory, antioxidant, hepatoprotective, antidiabetic, antidepressant, anxiolytic, antifungal, antibacterial, antiviral, and anticancer [8–10]. In the light of the above-mentioned facts, anilides of cinnamic acid were designed as multi-target compounds, synthesized using a modern microwave-assisted method and screened against a battery of bacterial, mycobacterial and fungal pathogens. In addition, anti-inflammatory activity and cytotoxicity were tested. The structure-activity relationships were investigated [11–16].
Materials and Methods
The synthesis and characterization of all discussed compounds was described previously [11,13,14,16].
Methodologies for testing biological activities were described previously [11–15].
Results and Discussion
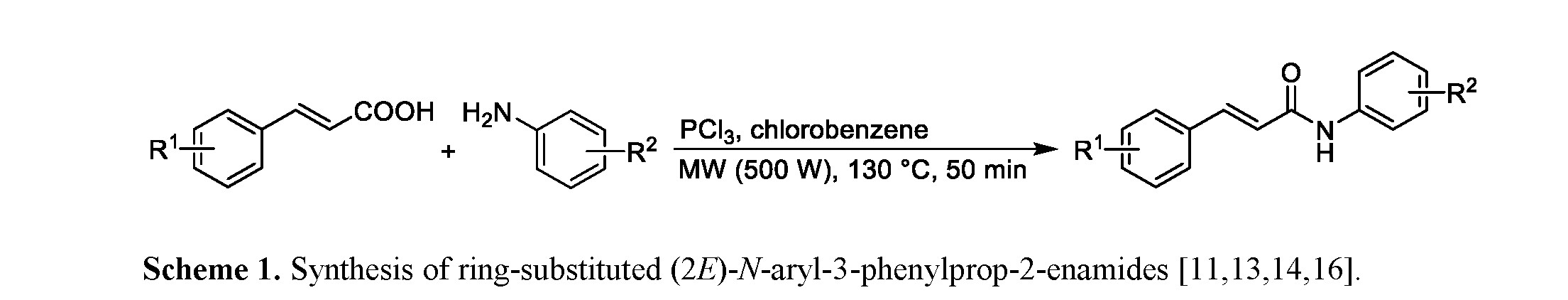
All compounds were synthesized from cinnamic acid in a microwave reactor: the carboxyl group was converted with phosphorus trichloride to acyl chloride, which then reacted with an appropriately substituted aniline to give the desired product [11,13–15], see Scheme 1.
Several investigated compounds showed antibacterial, antimycobacterial and antifungal activities, as well as anti-inflammatory potential comparable to or higher than those of clinically used drugs [11–14,16]. Selected biological effects of several investigated compounds are discussed below.
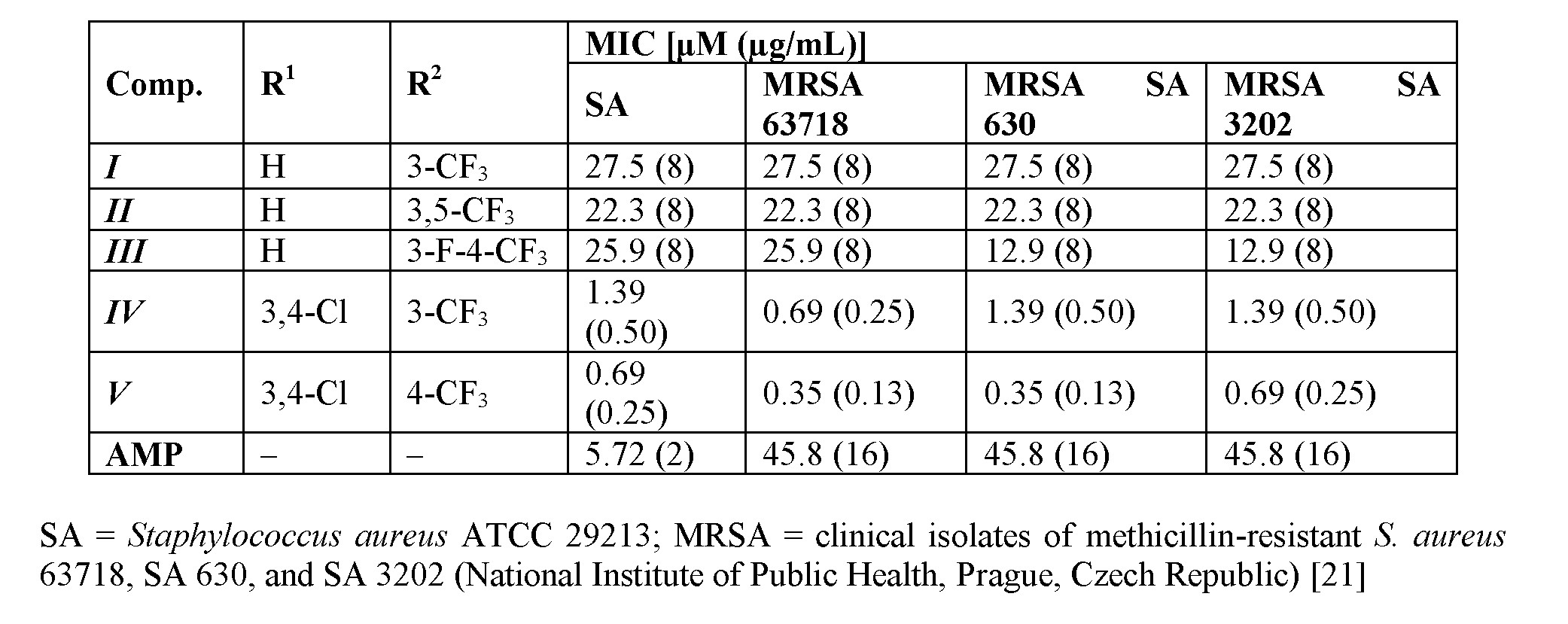
The cinnamic acid derivatives with the highest antistaphylococcal activity are presented in Table 1. Although the activity of cinnamic acid derivatives is known for a long time, the exact mechanism of action is still unknown. The most reported mechanism of action is interaction with plasmatic membrane. The compounds can cause disruption of the membrane, damage the membrane proteins, etc. [17–20]. There are also specific targets for cinnamic acid derivatives [20]. Nevertheless, it is possible that the wide spectrum of effects to cells is caused by the primary activity of the compounds, which is membrane destabilization [20].
Table 1. Selected ring-substituted (2E)-N-aryl-3-arylprop-2-enamides and their in vitro antistaphylococcal activities expressed as minimum inhibitory concentrations (MICs) compared to standard ampicillin (AMP).
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay can be used to assess cell growth by measuring respiration. The MTT measured viability of bacterial cells less than 70% [22] after the exposure to the MIC values of tested compound is considered as a positive result of this assay, because this low level of cell viability indicates the inhibition of cell growth by the inhibition of respiration [23]. It can be concluded that compound III showed a significant decrease in viability to 13.4%, which is far below the limit of 70% viability of S. aureus ATCC 29213 at the tested concentration equal to MIC (i.e., 25.9 µM (8 µg/mL) [14].
Compounds I and II were tested for their ability of synergic activity with clinically used antibacterial drugs, such as tetracycline, ciprofloxacin, and vancomycin. Both compounds showed additivity with vancomycin against MRSA SA 630 and SA 3202 [11]. Compound II had synergistic effect with ciprofloxacin against both tested strains. The effect of derivative II was also synergistic with tetracycline against MRSA SA 3202. The rest combinations with compound II had additive effect. Whereas compound II had a potential to increase the activity of all tested antibiotics, which have different mechanisms of actions and to which bacteria develop different resistance mechanisms, it can be expected that compound II acts by its own mechanism of action or increases the availability of the antibiotics by interaction with the membrane [11]. The dynamics of antibacterial activity was also evaluated against S.
51aureus ATCC 29213. Within the pre-test subcultivation aliquots on agar, compounds I–III showed bactericidal activity, i.e., minimal bactericidal concentrations were ≤4× MIC. These facts were verified using the time-kill curve assay. Also in the case of compounds I and III, the antibacterial activity was concentration-dependent very close to the bactericidal level [11,12,14]. The compounds were also able to inhibit the formation of a bacterial biofilm formed by S. aureus ATCC 29213. The lowest concentration of compound I, which inhibited ≥80% of the biofilm formation, was 8 µg/mL [11,12].
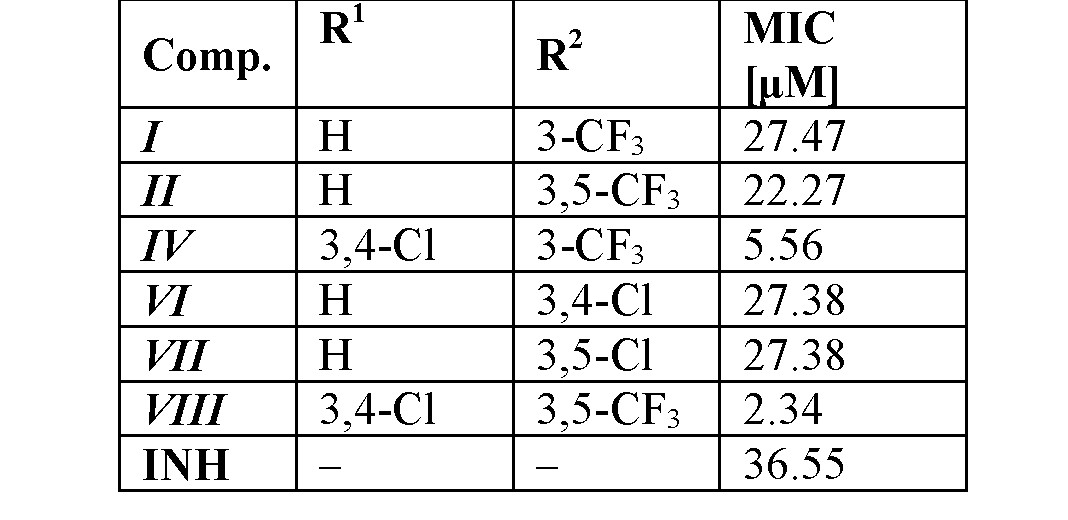
The selection of the most active compounds against Mycobacterium tuberculosis ATCC 25177/H37Ra [24] is in Table 2. Some of the compounds were also subjected to a standard MTT test (measuring the viability of M. tuberculosis H37Ra) [25] and showed less than 70% viability of M. tuberculosis H37Ra at the tested concentration equal to MICs [11]. Thus, it may be hypothesized that the mechanism of action of these ring-substituted cinnamanilides could be connected with the affection of mycobacterial energy metabolism [25,26], nevertheless, another possible site of action of the studied compounds in the mycobacteria cannot be excluded [27–30].
The overall results of this anti-infective screening indicate that derivatives substituted in positions C(3)', C(3,4)', C(3,5)' showed an increasing trend of activity. A bilinear dependence of activity on σAr (electronic properties of anilide ring) can be observed. The activity increases with the increasing electronwithdrawing effect to optimum σAr ca. 1 and then decreases with increasing values of the electronwithdrawing parameter [11,12,14].
Table 2. In vitro antitubercular activity (MICs) against M. tuberculosis H37Ra compared to isoniazid (INH).
The preliminary in vitro screening of the antiproliferative activity of the antimicrobially most effective on the phenyl ring unsubstituted cinnamic acid derivatives was performed using a Water Soluble Tetrazolium salts-1 (WST-1) assay kit [31] and the human monocytic leukemia THP-1 cell line. Almost all the tested compounds showed insignificant cytotoxic effect (IC50 >20 µM) [11,13,14].
The anti-inflammatory potential of the compounds to modulate the activity of pro-inflammatory transcription nuclear factor (NF)-κB was evaluated on THP1-Blue™ NF-κB cells stimulated with lipopolysaccharide (LPS). Most anilides significantly attenuate the LPS-induced NF-κB activation and were more active than pattern cinnamic acid. (2E)-N-[2-Chloro-5-(trifluoromethyl)phenyl]-3-phenylprop- 2-enamide (IX) showed the highest inhibitory effect, comparable with prednisone. Nuclear translocation of NF-κB after LPS stimulation affected by compound IX is visible in fluorescence microscope photographs. It is in agreement with observed inhibition of NF-κB activity and it can delineate the possible mechanisms of action [13,14]. As the activity of NF-κB is driven by the level of its inhibitor IκB and by the activity of several mitogen-activated protein kinases (MAPKs) [32], influence of compounds IX, (2E)-N-(2,5-dichlorophenyl)-3-phenylprop-2-enamide (X) and cinnamic acid on NF-κB and MAPKs expression was tested. Unfortunately, no effect on the IκBα levels and MAPKs activity was observed [13,14]. This discrepancy could be explained by a different mode of action. The tested anilides might act either via the inhibition of the nuclear translocation of NF-κB, or influence its binding to DNA, or act by epigenetic regulation (or a combination of more mechanism of actions) [33–35].
The overall results of anti-inflammatory potential indicate that monosubstituted anilides demonstrated lower inhibitory effect than the disubstituted ones. The position of the substituents on the anilide ring, lipophilicity, and bulkiness significantly affect the activity of the compounds. Disubstitutions
 52
52
of the C(2,5)' or C(2,6)' positions with bulky substituents are preferred. The electron-withdrawing properties of the anilide substituents appear to be more advantageous [13,14].
It should be also noted that the prepared compounds were additionally tested for their activity against plant pathogenic fungi Fusarium avenaceum (Fr.) Sacc. IMI 319947 and Bipolaris sorokiniana (Sacc.) Shoemaker H-299. The most effective antifungal agents did not show any in vivo plant toxicity performed on the leaves of Nicotiana tabacum var. Samsun [11].
Conclusions
A series of cinnamanilides as multi-target agents was designed and synthesized using a modern microwave-assisted method. Some compounds showed higher antimicrobial efficacy than clinically used drugs and demonstrated the ability to increase the effect of clinically used antibiotics. The compounds expressed bactericidal activity at concentrations close to the MICs and also inhibited the growth of staphylococcal biofilm and disrupted mature biofilm at concentrations close to the MICs. A significant decrease of bacterial cell metabolism (S. aureus viability) and mycobacterial cell metabolism (M. tuberculosis viability) was observed. Most tested compounds significantly attenuated LPS-induced NF- κB activation. Several compounds demonstrated activity comparable to prednisone. The mode of action is under investigation (nuclear translocation of NF-κB inhibition/DNA binding/epigenetic regulation or combinations). The design of a multi-target compounds combining antimicrobial and anti-inflammatory effects is a great challenge. Based on the results, it can be stated that for antimicrobial activity, substitution of the anilide ring in meta positions (optionally in combination with para) is preferred, while anti-inflammatory activity was observed for derivatives having an anilide ring substituted in ortho positions (optionally in combination with meta).
Acknowledgement
This study was supported by the Slovak Research and Development Agency (APVV-17-0373).
References
- Morphy, J.R. The Challenges of Multi-target Lead Optimization. Designing Multi-Target Drugs. Royal Society of Chemistry, London, 2012.
- Talevi, A. Multi-target pharmacology: possibilities and limitations of the “skeleton key approach” from a medicinal chemist perspective. Front. Pharmacol. 2015, 6, 205.
- Yang, T.; Sui, X.; Yu, B.; Shen, Y.; Cong, H. Recent advances in the rational drug design based on multi-target ligands. Curr. Med. Chem. 2020, 27, 4720–4740.
- Costantino, L.; Barlocco, D. Challenges in the design of multitarget drugs against multifactorial pathologies: a new life for medicinal chemistry? Future Med. Chem. 2013, 5, 5–7.
- Li, K.; Schurig-Briccio, L.A.; Feng, X.; Upadhyay, A.; Pujari, V.; Lechartier, B.; Fontes, F.L.; Yang, H.; Rao, G.; Zhu, W.; Gulati, A.; No, J.H.; Cintra, G.; Bogue, S.; Liu, Y.L.; Molohon, K.; Orlean, P.; Mitchell, D.A.; Freitas-Junior, L.; Ren, F.; Sun, H.; Jiang, T.; Li, Y.; Guo, R.T.; Cole, S.T.; Gennis, R.B.; Crick, D.C.; Oldfield, E. Multitarget drug discovery for tuberculosis and other infectious diseases. J. Med. Chem. 2014, 57, 3126–3139.
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3.
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd Ed. John Wiley & Sons, 2009.
- Gaikwad, N.; Nanduri, S.; Madhavi, Y.V. Cinnamamide: An insight into the pharmacological advances and structure-activity relationships. Eur. J. Med. Chem. 2019, 181, 111561.
- Sharma, P. Cinnamic acid derivatives: A new chapter of various pharmacological activities. J. Chem. Pharm. Res. 2011, 3, 403–423.
- Kos, J.; Strharsky, T.; Stepankova, S.; Svrckova, K.; Oravec, M.; Hosek, J.; Imramovsky, A.; Jampilek, J. Trimethoxycinnamates and their cholinesterase inhibitory activity. Appl. Sci. 2021, 11, 4691.
- Pospisilova, S.; Kos, J.; Michnova, H.; Kapustikova, I.; Strharsky, T.; Oravec, M.; Moricz, A.M.; Bakonyi, J.; Kauerova, T.; Kollar, P.; Cizek, A.; Jampilek, J. Synthesis and spectrum of biological activities of novel N-arylcinnamamides. Int. J. Mol. Sci. 2018, 19, 2318.
- Pospisilova, S.; Kos, J.; Michnova, H.; Strharsky, T.; Cizek, A.; Jampilek, J. N-Arylcinnamamides as Antistaphylococcal Agents. In Proceedings of the 4th International Electronic Conference on Medicinal Chemistry (ECMC-4), 2018, 5576, https://sciforum.net/manuscripts/5576/slides.pdf
(accessed on 06 October 2021).
- Hosek, J.; Kos, J.; Strharsky, T.; Cerna, L.; Starha, P.; Vanco, J.; Travnicek, Z.; Devinsky, F.; Jampilek, J. Investigation of anti-inflammatory potential of N-arylcinnamamide derivatives. Molecules 2019, 24, 4531.
- Kos, J.; Bak, A.; Kozik, V.; Jankech, T.; Strharsky, T.; Swietlicka, A.; Michnova, H.; Hosek, J.; Smolinski, A.; Oravec, M.; Devinsky, F.; Hutta, M.; Jampilek, J. Biological activities and ADMET- related properties of novel set of cinnamanilides. Molecules 2020, 25, 4121.
- Michnova, H.; Strharsky, T.; Kos, J.; Cizek, A.; Jampilek, J. Antistaphylococcal activity of polychlorinated N-arylcinnamamides. Proceedings: The 6h International Electronic Conference on Medicinal Chemistry (ECMC-6), November 1–30, 2020, 7403,
https://sciforum.net/paper/view/conference/7403 (accessed on 06 October 2021).
- Pindjakova, D.; Strharsky, T.; Kos, J.; Vrablova, L.; Hutta, M.; Jampilek, J. Study of ADMET descriptors of novel chlorinated N-arylcinnamamides. Chem. Proc. 2021, 3, 121.
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the action of selected essential oil components on gram negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595.
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561– 1568.
- Gill, A.O.; Holley, R.A. Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int. J. Food Microbiol. 2006, 3, 170–174.
- Langeveld, W.T.; Veldhuizen, E.J.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94.
- Zadrazilova, I.; Pospisilova, S.; Masarikova, M.; Imramovsky, A.; Ferriz, J.M.; Vinsova, J.; Cizek, A.; Jampilek, J. Salicylanilide carbamates: Promising antibacterial agents with high in vitro activity against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Pharm. Sci. 2015, 77, 197–207.
- International Organization for Standardization. ISO 10993-5:2009 Biological Evaluation of Medical Devices Part 5: Tests for in Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009; last revision 2017.
- Grela, E.; Kozłowska, J.; Grabowiecka, A. Current methodology of MTT assay in bacteria—A review. Acta Histochem. 2018, 120, 303–311.
- Zheng, H.; Lu, L.; Wang, B.; Pu, S.; Zhang, X.; Zhu, G.; Shi, W.; Zhang, L.; Wang, H.; Wang, S.; et al. Genetic basis of virulence attenuation revealed by comparative genomic analysis of Mycobacterium tuberculosis strain H37Ra versus H37Rv. PLoS ONE 2008, 3, e2375.
- Bueno, J. Antitubercular in vitro drug discovery: Tools for begin the search. In Understanding Tuberculosis-New Approaches to Fighting against Drug Resistance, Cardona, P.J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 147–168.
- Zumla, A.; Nahid, P.; Cole, S.T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug Discov. 2013, 12, 388–404.
- Chen, Y.L.; Huang, S.T.; Sun, F.M.; Chiang, Y.L.; Chiang, C.J.; Tsai, C.M.; Weng, C.J. Transformation of cinnamic acid from trans- to cis-form raises a notable bactericidal and synergistic activity against multiple-drug resistant Mycobacterium tuberculosis. Eur. J. Pharm. Sci. 2011, 43, 188–194.
- De, P.; Koumba, Y.G.; Constant, P.; Bedos-Belval, F.; Duran, H.; Saffon, N.; Daffe, M.; Baltas, M. Design, synthesis, and biological evaluation of new cinnamic derivatives as antituberculosis agents. J. Med. Chem. 2011, 54, 1449–1461.
- De, P.; Veau, D.; Bedos-Belval, F.; Chassaing, S.; Baltas, M. Cinnamic derivatives in tuberculosis. In Understanding Tuberculosis-New Approaches to Fighting against Drug Resistance; Cardona, P.J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 337–362.
- Adeniji, S.E.; Uba, S.; Uzairu, A. Quantitative structure–activity relationship and molecular docking of 4-alkoxy-cinnamic analogues as anti-mycobacterium tuberculosis. J. King Saud Uni. Sci. 2020, 32, 67–74.
- ROCHE, 2021. Cell proliferation reagent WST-1. Roche Diagnostics GmbH, Mannheim, Germany. Available online: https://www.sigmaaldrich.com/content/dam/sigma-
aldrich/docs/Roche/Bulletin/1/cellprorobul.pdf (accessed on 06 October 2021).
- Hoesel, B.; Schmid, J.A. The complexity of NF-kappa B signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86.
- D'Acquisto, F.; May, M.J.; Ghosh, S. Inhibition of nuclear factor kappa B (NF-kB): An emerging theme in anti-inflammatory therapies. Mol. Interv. 2002, 2, 22–35.
- Liu, T.; Zhang, L.Y.; Joo, D.; Sun, S.C. NF-kappa B signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023.
- Wierda, R.J.; Geutskens, S.B.; Jukema, J.W.; Quax, P.H.A.; van den Elsen, P.J. Epigenetics in atherosclerosis and inflammation. J. Cell. Mol. Med. 2010, 14, 1225–1240.