Annotation
The main features of electrochemical deposition of the coatings based on Ni - W alloy in the pulse current mode using pyrophosphate electrolyte was studied. Two electrolytes with pH of 8.7 and 9.5 were used. The deposition was carried out with the current density varied in the range of 0.01 - 0.1 Aciii2. and the duty cycle (the relative pulse duration) was changed within 20 - 100 %. The surface morphology, elemental and phase composition of the coatings were studied by SEM, EDS and XRD. The experimental conditions allowing to reach maximum of the Faradaic efficiency and W - content in the coatings are determined. It was found that the pulse current mode enabled to fabricate Iiigh - quality coatings without cracks at a thickness greater than 6 pm.
Introduction
Electrodeposited metal and alloy coatings are widely employed in order to increase surface hardness, electroconductivity, wearability, corrosive and electroerosive resistance, to provide necessary decorative properties, etc. Among them molybdenum and tungsten alloys with metals of the iron group, in particular Ni-W alloys, are regarded as very promising electrolytic coatings possessing high operating characteristics, especially electroerosive resistance [1 - 3]. Microhardness of these alloys depends on the relative W - content and can reach 4.5 - 7 GPa. After the heat treatment its value increases to 9 - 10 GPa.
Binary Ni-W coatings have prospects to become a good substitution for hard chromium plating. As compare with Cr coatings, Ni-W coatings keep hardness at elevated temperatures and exhibit higher corrosive and wear resistances [4, 5]. Besides, deposition of Ni-W coatings is nontoxic and environmentally friendly process [6, 7], and it is less power - consuming [8] as against Cr electroplating technology.
The field of application of Ni - W electrodeposited coatings is vast enough [9]. For example, the proper corrosion resistance of these alloys has made their application possible as hard coatings [10 - 19]. It is known [20 - 22] that Ni-W alloys exhibit catalytical activities [20 - 22] and can be employed as catalysts for oxidation of exhaust gases of internal combustion engines.
In electrical contacts, it is necessary to have effective barrier layers against corrosion and diffusion. Because nanocrystalline Ni-W can improve the corrosion resistance, minimize contact wear, and significantly reduce the formation of intermetallic compounds, resulting from interlayer diffusion at interfaces, these alloys with 15.8 at. % of tungsten can be considered as reasonably effective barriers for electronic/electrical contacts [23].
One of the main problem of electroplating technology is a high level of internal stresses in the deposited coatings causing their damaging (cracks). Using water - based electrolytes for Ni-W electroplating, one can observe significant hydrogen release resulting in the distortion of coating structure due to its hydrogen saturation, which provokes stress cracking of coatings. These cracks significantly influence on the operating characteristics of coatings, particularly on their corrosion resistance [9]. To avoid crack development, different methods are used like introduction of special additives to electrolytes, application of pulse current mode, employing of third coprecipitating metal, and deposition under supergravitation conditions [24].
In [24], an influence of the electrolytes with different ligands (citrate, glycine, triethanolamine, and their combinations) as well as the regimes of electrochemical deposition (DC and pulse mode with reverse current) on the level of internal stresses in Ni - W coatings were studied. It was shown that in all cases an internal stress in the coatings increases with the time passed from the beginning of deposition. To decrease internal stress, complex electrolyte contained three abovementioned ligands in equal proportions was proposed to use. Pulse - reverse current mode somewhat decreased such stresses but did not prevent their development with the course of time. At the same time, an adding of 1,3,6 naphthalene trisulfonic acid and insertion of chloride ions in electrolyte by means of partly replacing of nickel sulfite for its chloride compound along with application of the pulse - reverse current mode significantly reduce the probability of cracks development in coatings. However, in that case the coatings exhibit high surface roughness and rather low W - content (ca. 7 - 9 at. %), which limits their range of application.
It was shown [25] that electrochemical deposition of Ni - W alloys from citrate electrolyte under the conditions of high gravitation (within the range of IOl - 256 g) suppress the cracks development. An effective removing of hydrogen bubbles off the coating surface by means of increased the Archimedean force is considered as the main mechanism responsible for an improving of the coatings quality.
Comparative study on electrochemical deposition of Ni - W alloys from citrate electrolyte under DC and pulse current (PC) modes was carried out in [26]. It was revealed that PC mode promotes an increase of W content as compared to DC mode, and significant decrease of the surface roughness of coatings was achieved under the duration of cathode pulse of 1 millisecond (at the ratio of cathode pulse duration to the pause as 1/10).
Well - proved citrate electrolytes on base of organic ligands glycine and triethanolamine are widely employed for the fabrication of Ni - W coatings. However, their use is not always possible due to the aggressive interaction of citrate and citric acid with sub strate materi al s.
The main disadvantages of these electrolytes are the following: deterioration of the coatings adhesion due to the formation in electrolyte the products of anodic oxidation of ligands ions;
presence in the coatings carbon - containing organic impurities, which can cause carbon diffusion towards the surface of coatings;
rather low stability of the electrolyte composition.
Among various electrolytes with inorganic ligands pyrophosphate electrolytes can be employed for the production of coatings on base of refractory metals, including Ni-W coatings. The main advantages of such electrolytes are their stability, an absence of anodic oxidation of ligands ions, high scattering power, an ability to produce «smooth - faced» coating (of low surface roughness), carbonless composition of electrolytes, and environmental safety. However, the features of electrochemical deposition of Ni - W alloys from pyrophosphate electrolytes are insufficiently investigated. For example, in [27, 28] the deposition of these alloys in DC mode was studied but the presence of surface cracks in coatings was revealed.
The present work purposes the studying electrochemical deposition of N - W binary alloys by using pyrophosphate electrolytes in pulse current mode and, basing on the results of this study, the revealing of main features of the fabrication of crack - free coatings.
Experimental
-
- Plating bath chemistry
Binary N-W alloy was electrochemically deposited from pyrophosphate - ammonium electrolyte [28] ContainingNiSO4AH2O (nickel sulfate hexahydrate) 0.2 M, NasWCL (sodium tungstate) 0.2 M, K4P2O7 (potassium pyrophosphate) 0.6 M, and (NH4)2SO4 (ammonium sulfate) 0.15 M. All these components are dissolved in bi - distilled water with resistivity of 1 MOhm.cm. Two electrolytes with different pH, namely, 8.7 and 9.5, were used. Such choice is determined by the range of electrolyte stability (8.5 - 10.0) and by significantly difference in the ligand content of electrolytes depending on pH value [28]. This value was adjusted by means of adding H2SO4 (0.1 M solution) and NaOH (0.1 M solution). Pyrophosphate ions P2O74 form complex ions with Ni2+ ions of Ni(P2O7)26 composition, and an addition of NH4+ ions significantly increases the Faradaic efficiency [27]. The volume in each experiment was 250 ml.
-
- Operating conditions
A usual two - electrode cell was used in our experiments. As substrates, oxygen - free copper Cu - DHP plates by size of 1x2 cm2 with a thickness of 1 mm were employed. Substrates were polished by means of a felt circle without any abrasive and then were degreased in the solution containing NaOH and Na2CO3 with concentration of IOO g-1 1 for each component at the temperature of 70 °C. Before electrochemical deposition substrates were immersed in 15 % solution of HCl for 30 s and then were washed in bi - distilled water. As an anode, a platinum plate of a 4 cm2 in square was used. The current density was ranged within 0.01 - 0.1 A.cm 2, and both DC and PC modes were applied. The pulse repetition period did not change and was 100 ms; the cathode pulse duration varied within 20 - 50 ms, and the pause duration are chosen in the range of 50 - 80 ms. A potentiostat IPC Pro ЗА by Volta (Saint - Petersburg, Russian Federation) was employed to provide the electrical currents during electrodeposition.
The electrolyte’s temperature in our experiments was 55 ± 1 °C. It was provided by means of a magnetic stirring with heating C - MAG HS 7 digital by IKA (USA) using the feedback via a temperature sensor PTlOOO by Thermometries (USA). Stirring of the electrolyte was not used.
-
- Characterization techniques
Microstructural images were collected using a scanning electron microscope JSM - 66IOLV by JEOL (Japan) equipped with an energy dispersive X - ray microanalyzer INCA X - MAX by Oxford Instruments (England) allowed determining quantitative elemental composition of the coatings. We used a 30 keV acceleration potential in our experiments. The thickness of coatings was determined by means of cross - section microstructural measurements on SEM images. Relative accuracy of these measurements was estimated within the range of ± 3.5 %. Phase composition of the coatings was studied by X - ray diffractometer D8 Discover by Bruker (Germany) at the Research Center «Physics and technology of micro - and nanostructures» by the Institute for Physics of Microstructures Russian Academy of Science (Nizhny Novgorod, Russian Federation).
During our experiments more than 150 coatings of different thickness using two electrolytes with pH of 8.7 and 9.5 were produced and studied. The electrochemical deposition was carried out in DC and PC modes for different current densities.
Below we consider the chemical reactions taking place in the course of Ni - W deposition from pyrophosphate electrolyte [27, 28].
The nickel reduction occurs via three - stage reaction:
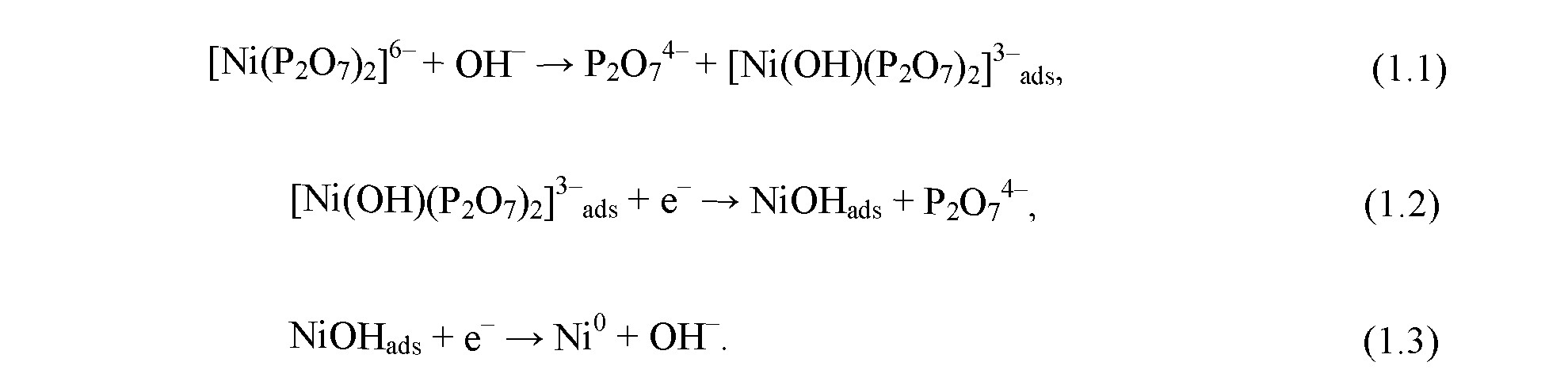 At the first stage, complex pyrophosphate ion [Ni(P2O2)2]6 interacts with hydroxide ion OH which results in the formation of complex ion [Ni(OH)(P2O2)2]3 . This ion is adsorbed on the surface of the growing coating. At the second stage, it is reduced to NiOH, which in turn is reduced to metallic nickel.
At the first stage, complex pyrophosphate ion [Ni(P2O2)2]6 interacts with hydroxide ion OH which results in the formation of complex ion [Ni(OH)(P2O2)2]3 . This ion is adsorbed on the surface of the growing coating. At the second stage, it is reduced to NiOH, which in turn is reduced to metallic nickel.
In accordance with [28], coprecipitation of tungsten occurs via the chemical reaction resulting in the formation of cluster heterometallic compound containing Ni — W bond:
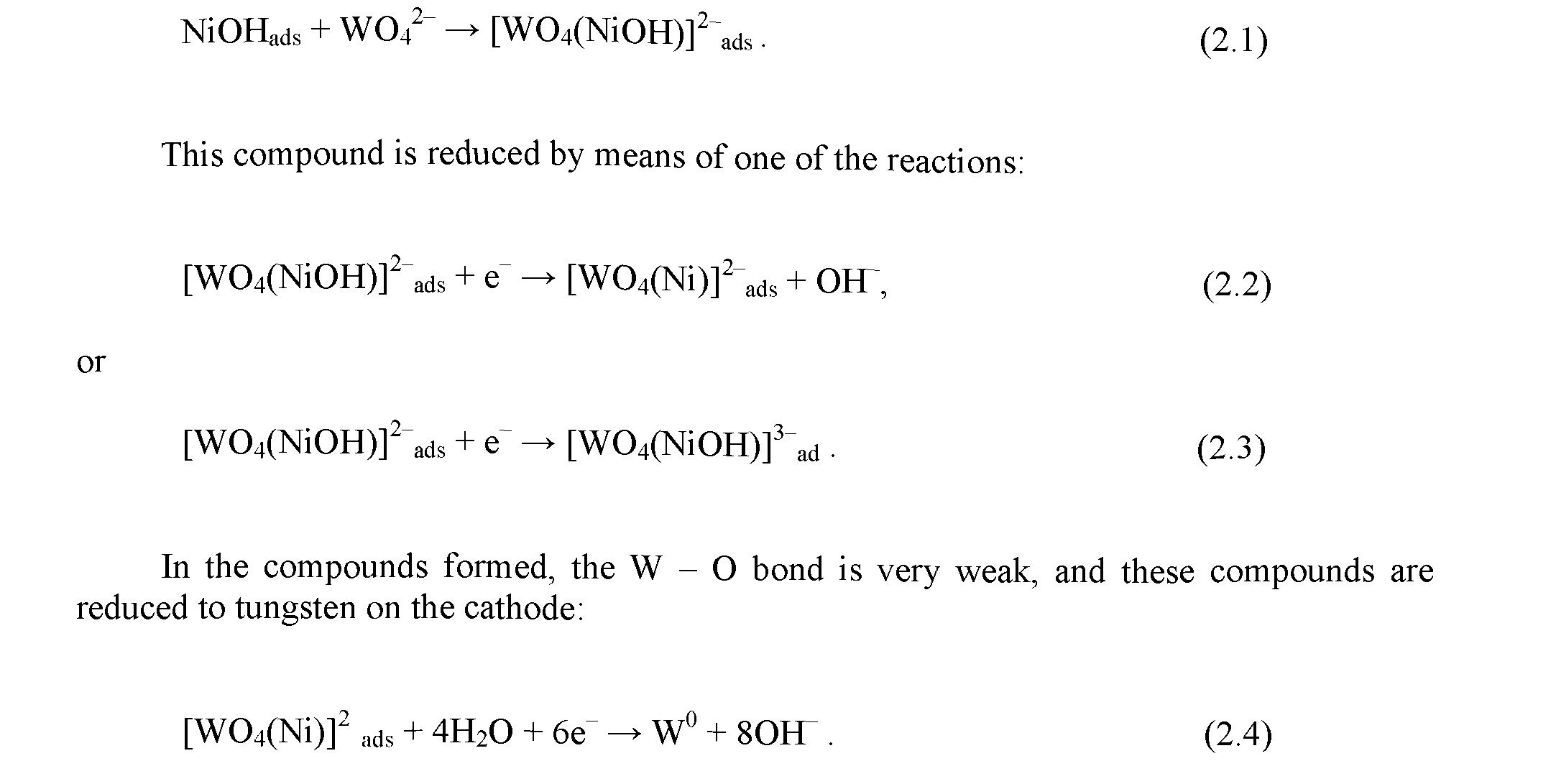 Representative scanning electron microscopy (SEM) images of Ni - W coatings produced in DC mode with different current densities are presented in Fig. 1. Inserts in the upper right corner in both panels of this figure show energy dispersive X - ray spectra (EDS). The pH in both cases was 9.5, and a thickness of the coatings was ca. 6 pm. On these figures, one sees a rather dense net of microcracks on the surface resulting either due to hydrogen embrittlement or from residual stresses [29, 30]. This net is formed in stochastic manner and coincides with globule boundaries, but only for some fragments. Also, the number of cracks and W - content increase with an increase in the current density. As mentioned in [1, 2, 28] such coating of a thickness bigger than 5 pm is practically impossible to prepare without cracks using DC mode.
Representative scanning electron microscopy (SEM) images of Ni - W coatings produced in DC mode with different current densities are presented in Fig. 1. Inserts in the upper right corner in both panels of this figure show energy dispersive X - ray spectra (EDS). The pH in both cases was 9.5, and a thickness of the coatings was ca. 6 pm. On these figures, one sees a rather dense net of microcracks on the surface resulting either due to hydrogen embrittlement or from residual stresses [29, 30]. This net is formed in stochastic manner and coincides with globule boundaries, but only for some fragments. Also, the number of cracks and W - content increase with an increase in the current density. As mentioned in [1, 2, 28] such coating of a thickness bigger than 5 pm is practically impossible to prepare without cracks using DC mode.
-
- An influence of the conditions of electrochemical deposition on the Faradaic efficiency and W - content in the coatings
 To study an influence of the cathode current density on the Faradaic efficiency (FE) and W - content in coatings, we fabricated a set of the coatings under the conditions of constant charge passed through electrolyte.
To study an influence of the cathode current density on the Faradaic efficiency (FE) and W - content in coatings, we fabricated a set of the coatings under the conditions of constant charge passed through electrolyte.
where is the component of the FE for W, is the component of the FE for Ni, Ew is the electrochemical equivalent for the six - valent W equal 0.318 10 3 g/C, Em is the electrochemical equivalent for the bivalent Ni equal 0.304-10 ‘ g/C, Am is the mass of coating (in g), ww is the relative mass content of W in alloy, w is the relative mass content of Ni in alloy, q is the charge passed through electrolyte calculated as
where Zis the working current, and t is the time of deposition.
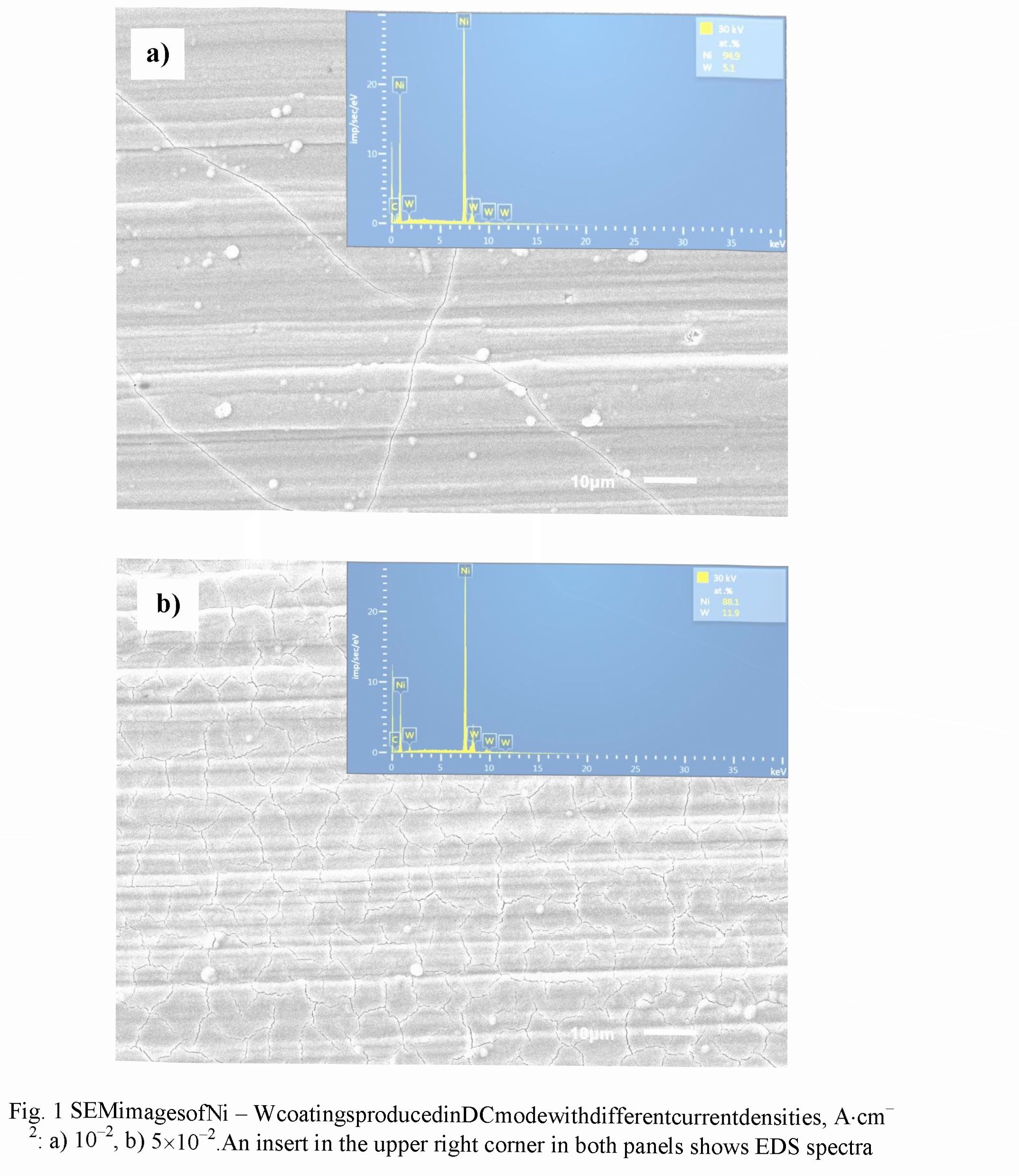
Figs. 2 and 3 exhibit the dependencies of the Faradaic efficiency and W-content measured in the different current modes for electrolytes with the pH of 8.7 and 9.5, respectively. Relative accuracies of the FE and W - content measurements were ± 2.5 % and ±1.5 %, respectively.
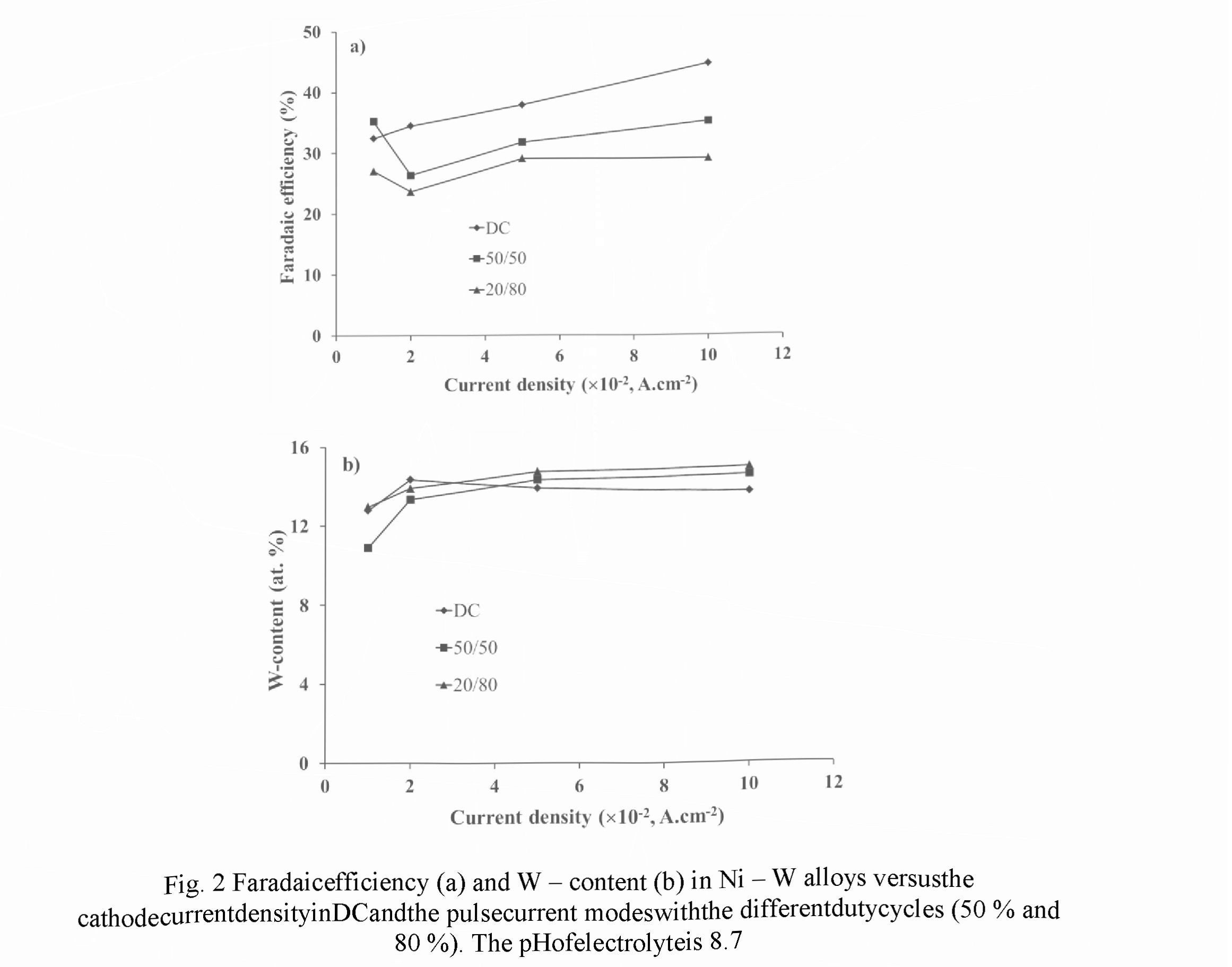
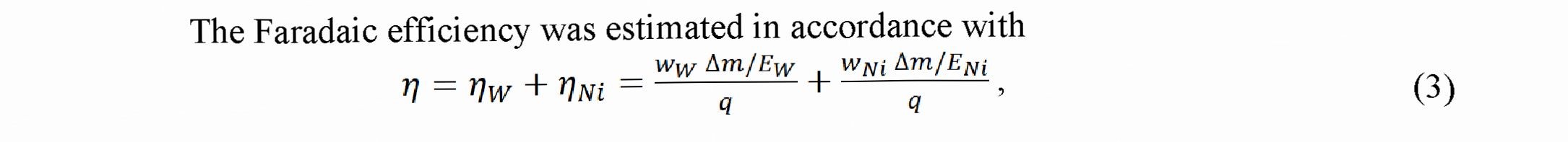
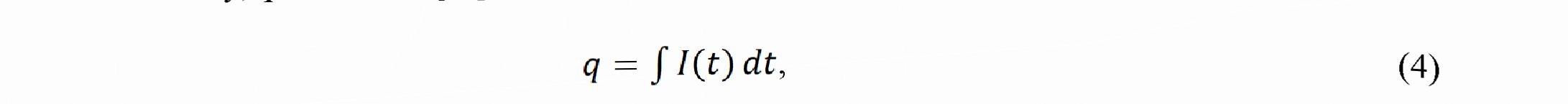
For electrolyte with pH 8.7 in PC mode a decrease in the Faradic efficiency of 20 - 30 % was observed in comparison with DC mode (Fig. 2a). Within the current density of 0.02 -0.1 A.cm " for both modes the monotonic rise of the FE values was revealed, but a decrease of the duty cycle (the relative pulse duration) results in the decreasing of FE. Such dependencies can be explained by the fact that for pH ranged from 8.5 to 9.5 the Faradaic efficiency exhibits an increase with the increasing of pH value, and the current density rise provokes the rise of the pH value in the near - cathode area. Contrariwise, a decrease of the duty cycle reduces pH value of electrolyte at this area.
For both modes the weak dependence of W - content on the cathode current density was observed (Fig. 2b). In comparison with DC mode, in the pulse current mode a certain increase in W - content occurs in alloy. At the same time, a decrease of the duty cycle leads to an increase in W - content, which can be explained by the restoration of the concentration of W - containing ligand ions in the near - cathode layer due their diffusion during the pauses between pulses.
An evident difference of the dependencies presented in Figs. 2 in the range of small densities of the cathode current (0.01 - 0.02 A.cm 2) as against the current range discussed above indicates the transition to the other kinetics of electrochemical reactions occurred in that range.
For electrolyte with pH of 9.5 the FE dependencies showed more complex character (Fig. 3a). However, for both current modes they look similar: a decrease in the range of 0.01 - 0.02 A.cm ", then, a rise in the range of 0.02-0.05 A.cm 2, and, finally, again a decrease in the range of 0.05 - 0.1 A.cm . Two contrary reasons can be responsible for the maximum in the intermediate current range: on one hand, an increase of the current density results in a rise of pH value in the near - cathode layer, which increased the FE, and, on the other hand, an increase in the current density leads to the depletion of the near — cathode layer and to decrease in the concentration of ligand metal ions in it, which in turn decreases the FE. The use of PC mode for a given electrolyte leads to an increase in the Faradaic efficiency, and its value also increases with the decreasing of the duty cycle. It can be explained by more effective restoration of the concentration of ligand metal ions in the near - cathode area due to diffusion occurring during the pauses between pulses.
For PC mode in the current density ranged within 0.03 - 0.1 AcrrF2, a higher W - content in the coating is achieved as compared to DC mode (Fig. 3b). A decrease in the duty cycle results in an increase of this content, and for the given experimental conditions maximum W - content of 8.2 at.% is provided at the duty cycle of 20 % and the cathode current density of 0.05 A.cm 2.
To compare the results obtained for electrolytes with pH 8.7 and 9.5, one can see that lower pH value enables higher W - content in alloy, namely, 12 - 14.5 at. % as against 4.5 - 8.5 at.%, respectively. However, the FE of electrolyte with pH 9.5 is higher than in case of pH 8.7, in 2 — 3 times for the pulse current mode. For DC mode the more complex character of the FE dependencies is revealed: in the range of 0.01 - 0.05 A.cm 2 the FE for pH 9.5 increases in 1.2 - 1.6 times, and, on the contrary, in the range of 0.08 - 0.1 A.cm 2 it decreases in 1.4 - 1.8 times.
-
- An influence of the conditions of electrochemical deposition on the morphology and microstructure of the coatings
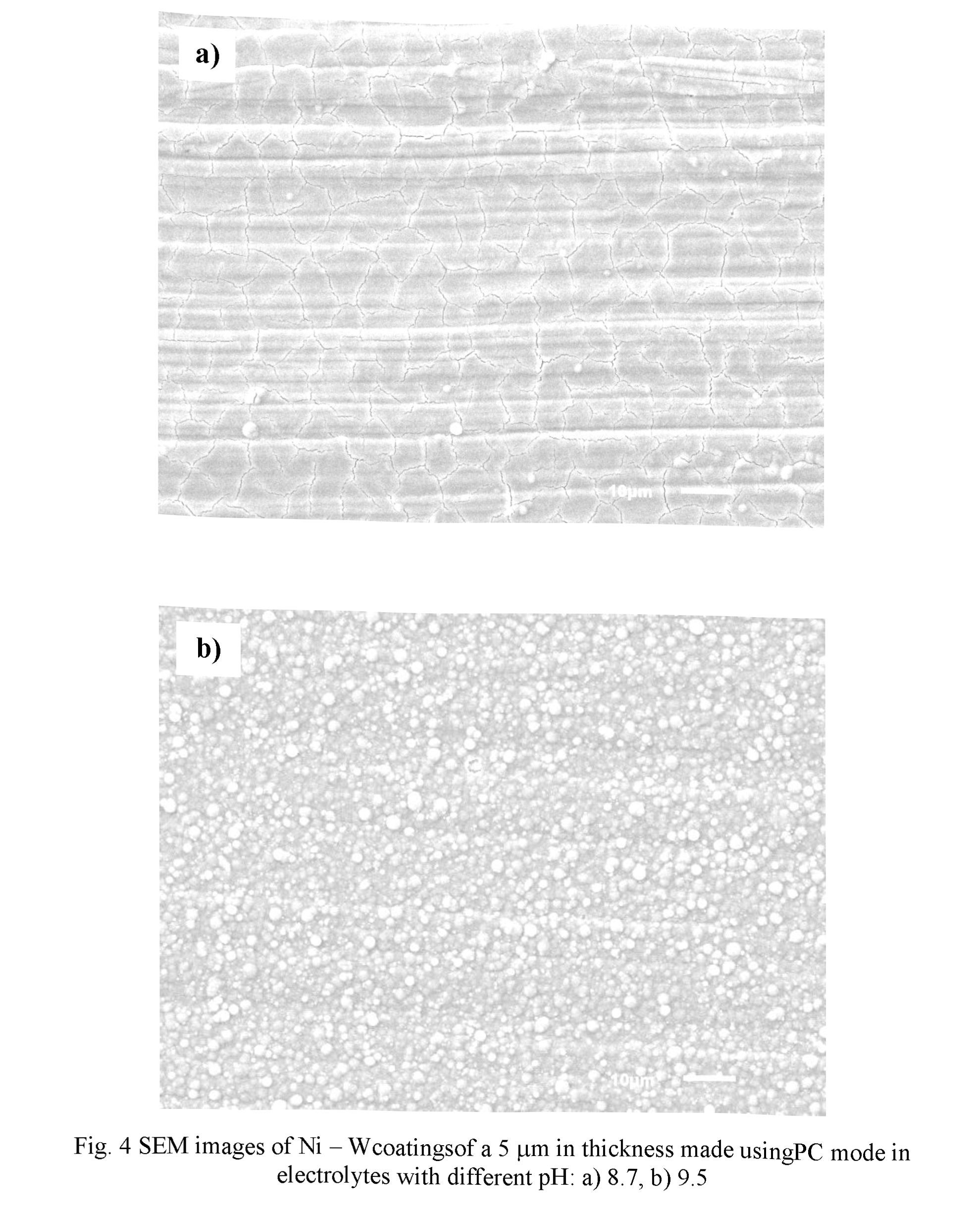
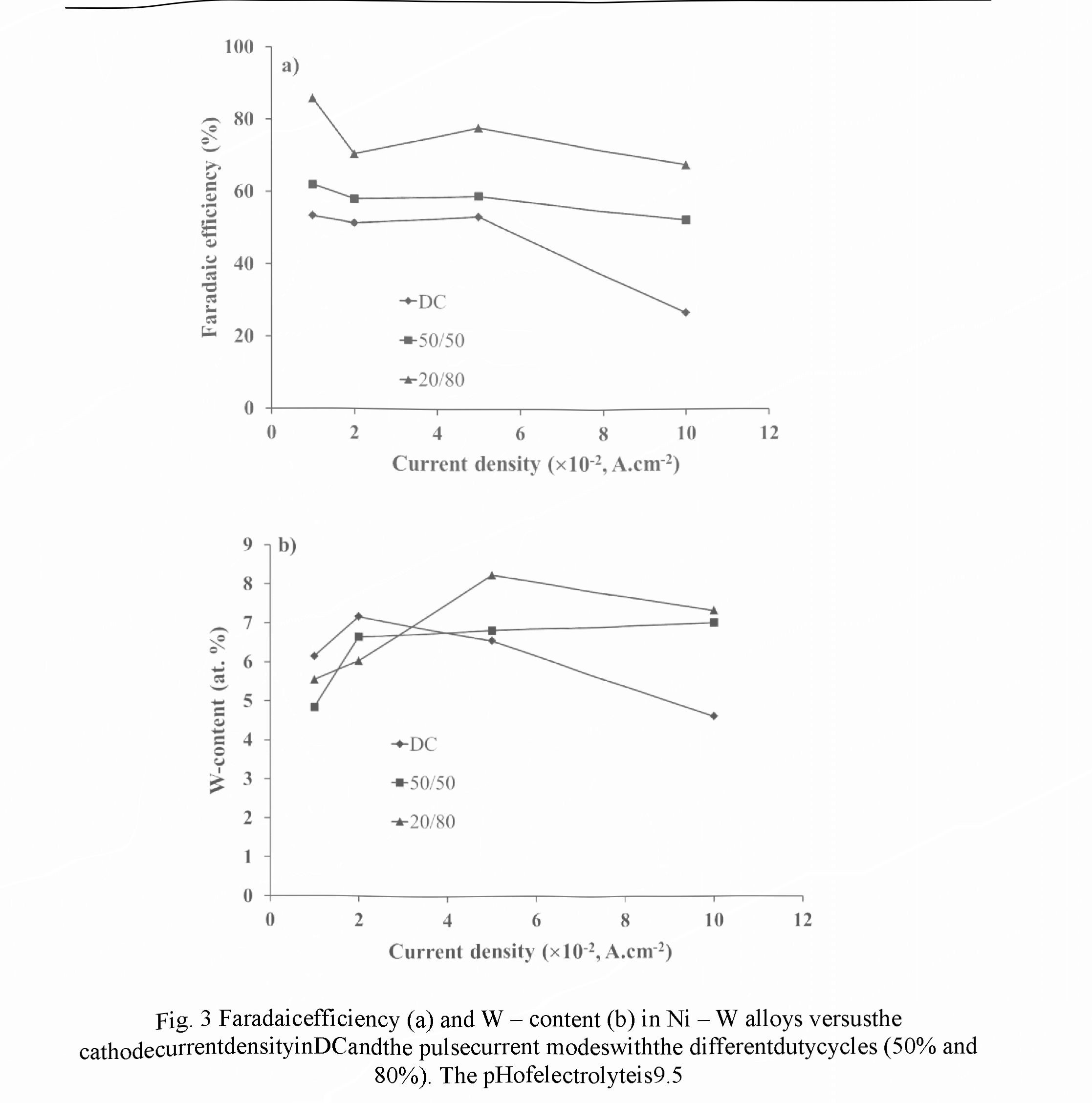 Fig. 4 shows representative SEM images of N - W coatings made using electrolytes with pH 8.7 (Fig. 4a) and pH 9.5 (Fig. 4b). In both cases the duty cycle was chosen to be 20 %, and the current density was 0.05 A.cm-2. The coatings possessed characteristic metallic luster and good adhesion to the substrate. Surface microstmcture of the coatings differs from each other. The coating produced in electrolyte with pH 8.7 (Fig. 4a) exhibits smaller numbers of separately located globules, the dimension of which is ranged within 0.5 - 3 pm, and in case of electrolyte with pH 9.5 (Fig. 4b) the globular structure is more pronounced.
Fig. 4 shows representative SEM images of N - W coatings made using electrolytes with pH 8.7 (Fig. 4a) and pH 9.5 (Fig. 4b). In both cases the duty cycle was chosen to be 20 %, and the current density was 0.05 A.cm-2. The coatings possessed characteristic metallic luster and good adhesion to the substrate. Surface microstmcture of the coatings differs from each other. The coating produced in electrolyte with pH 8.7 (Fig. 4a) exhibits smaller numbers of separately located globules, the dimension of which is ranged within 0.5 - 3 pm, and in case of electrolyte with pH 9.5 (Fig. 4b) the globular structure is more pronounced.
231
Electrochemical deposition of crack - free coatings
on base of NI - W binary alloy
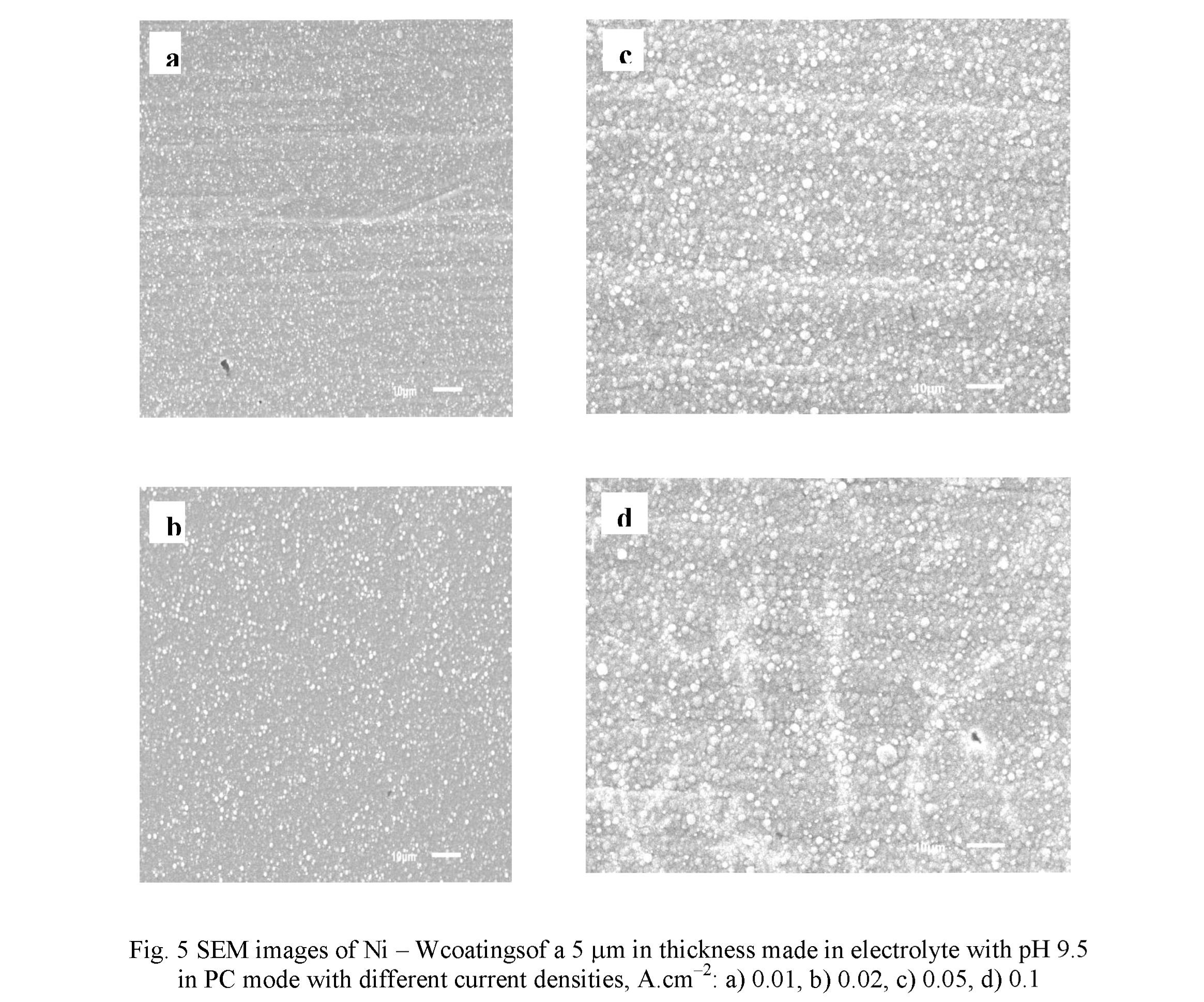
An influence of the cathode current density on coating microstructure was studied for the coating produced in electrolyte with pH 9.5 (Fig. 5). An increase of the current density leads to an enlargement of the lateral dimensions of globules, from the values of 0.3 - 1.5 pm at 0.01 A.cm" to 1 - 3 pm at 0.05 A.cm 2. At that, the number of globules slightly decreases. With a great degree of probability, this is due to the local amplification Ofelectric field and, correspondingly, the current density on the surface of globules, which ensures a rapid growth of their dimensions [27]. It can be mentioned that noticeable growth of globule dimensions ceases after the reaching the current density of 0.05 A.cm 2 (Figs. 5c, d). For the coatings produced in electrolyte with pH 8.7 such a regularity was not found. Also, the duty cycle does not contribute in a great extent in the development of surface microstructures.
 To study phase composition and revealing of oxides and intermetallic inclusions in Ni - W coatings, X - ray diffraction (XRD) analysis was carried out. The samples were prepared in PC mode with the different current densities using electrolytes of pH 8.7 and 9.5. The lines of Cu (from the substrate) and of the solid solution of W in Ni with f.c.c. lattice were observed, which is an agreement with the data published in [28, 31]. The lines of the solid solution were significantly broadened due to small dimensions of crystalline grains. Using these data, an average size of the regions of coherent scattering of Ni - W phase was estimated to be 8 - 10 nm for all the samples studied.
To study phase composition and revealing of oxides and intermetallic inclusions in Ni - W coatings, X - ray diffraction (XRD) analysis was carried out. The samples were prepared in PC mode with the different current densities using electrolytes of pH 8.7 and 9.5. The lines of Cu (from the substrate) and of the solid solution of W in Ni with f.c.c. lattice were observed, which is an agreement with the data published in [28, 31]. The lines of the solid solution were significantly broadened due to small dimensions of crystalline grains. Using these data, an average size of the regions of coherent scattering of Ni - W phase was estimated to be 8 - 10 nm for all the samples studied.
X - ray diffraction patterns shown in Fig. 6 were obtained for the N - W coatings produced in PC mode using electrolytes with pH 8.7 (the sample #94) and pH 9.5 (the sample #96). The current density was 0.05 A.cm-2 for both samples. One can see that the lines related to Ni - W phase are different for these samples, and the shift of the lines is greater for those measured at bigger diffraction angles. In our opinion, this fact indicates the difference in the lattice spacing and in Nii-x-Wx composition too, both of which, as found above, depend on pH of electrolyte. Also, no lines indicated the presence of the other crystalline phases (oxides, nitrides, intermetals) were observed.
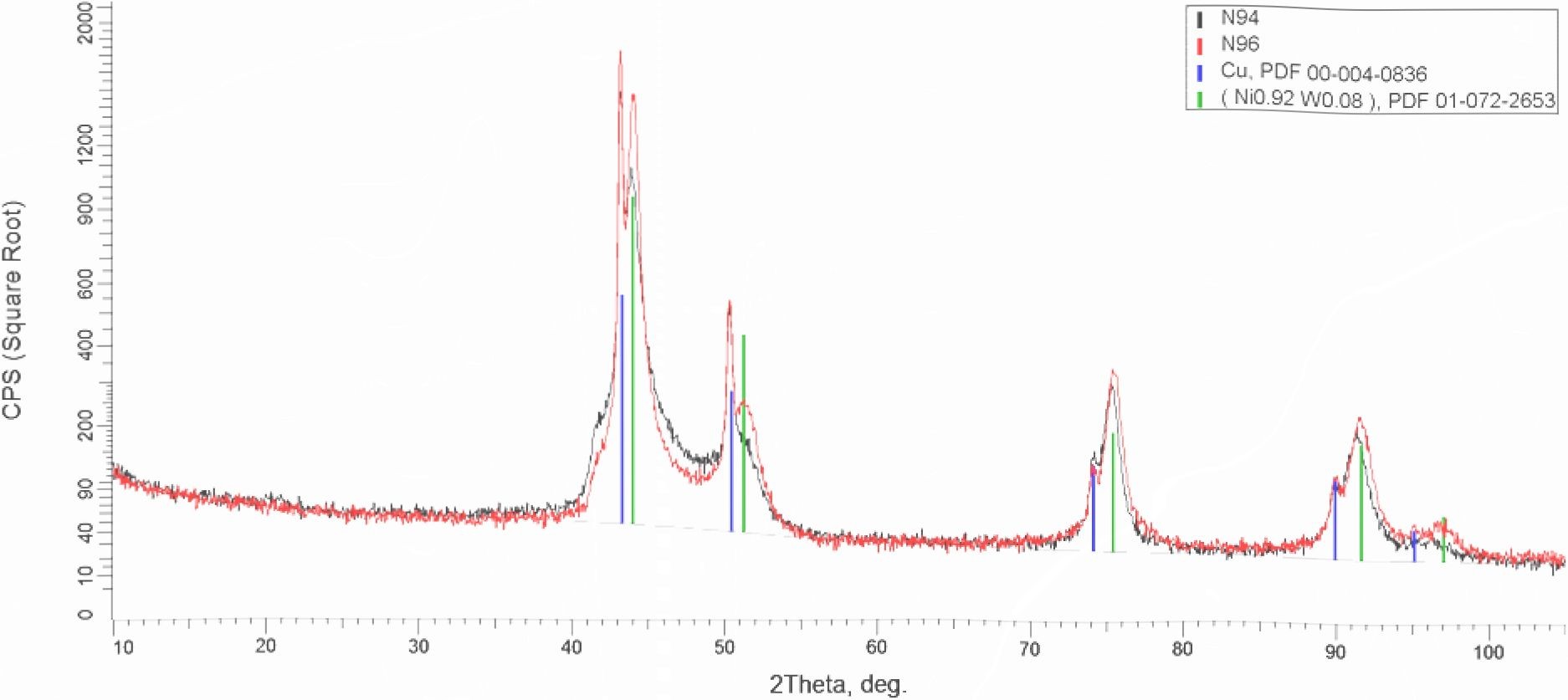
Fig. 6 X - ray diffraction patterns obtained for N - W coatings produced in the pulse current mode using electrolytes with pH 8.7 (the sample #94) and pH 9.5 (the sample #96). Thickness of the coatings in both cases was 5 pm
-
- Preparation of crack -free coatings with maximum thickness
To study the preparation of crack - free coatings with maximum thickness, a set of samples in identical experimental conditions with a stepwise increasing deposition time for each subsequent sample was fabricated. The beginning of crack formation was determined from SEM images. It was found that in the pulse current mode the thickness of crack - free coatings is significantly (in a few times) bigger than in DC mode. Fig. 7 shows the maximum coating thickness versus the duty cycle (the duty cycle of 100 % corresponds to DC mode). The experiments were conducted with the constant current density of 0.05 A.cm 2 for electrolytes with pH 8.7 and 9.5, and the thickest crack - fee coating was obtained for electrolyte with pH 9.5 at the duty cycle of 20 %. This result can be explained by lower hydrogen content in the coating due to its effective removal off the coating surface during the pauses between pulses.
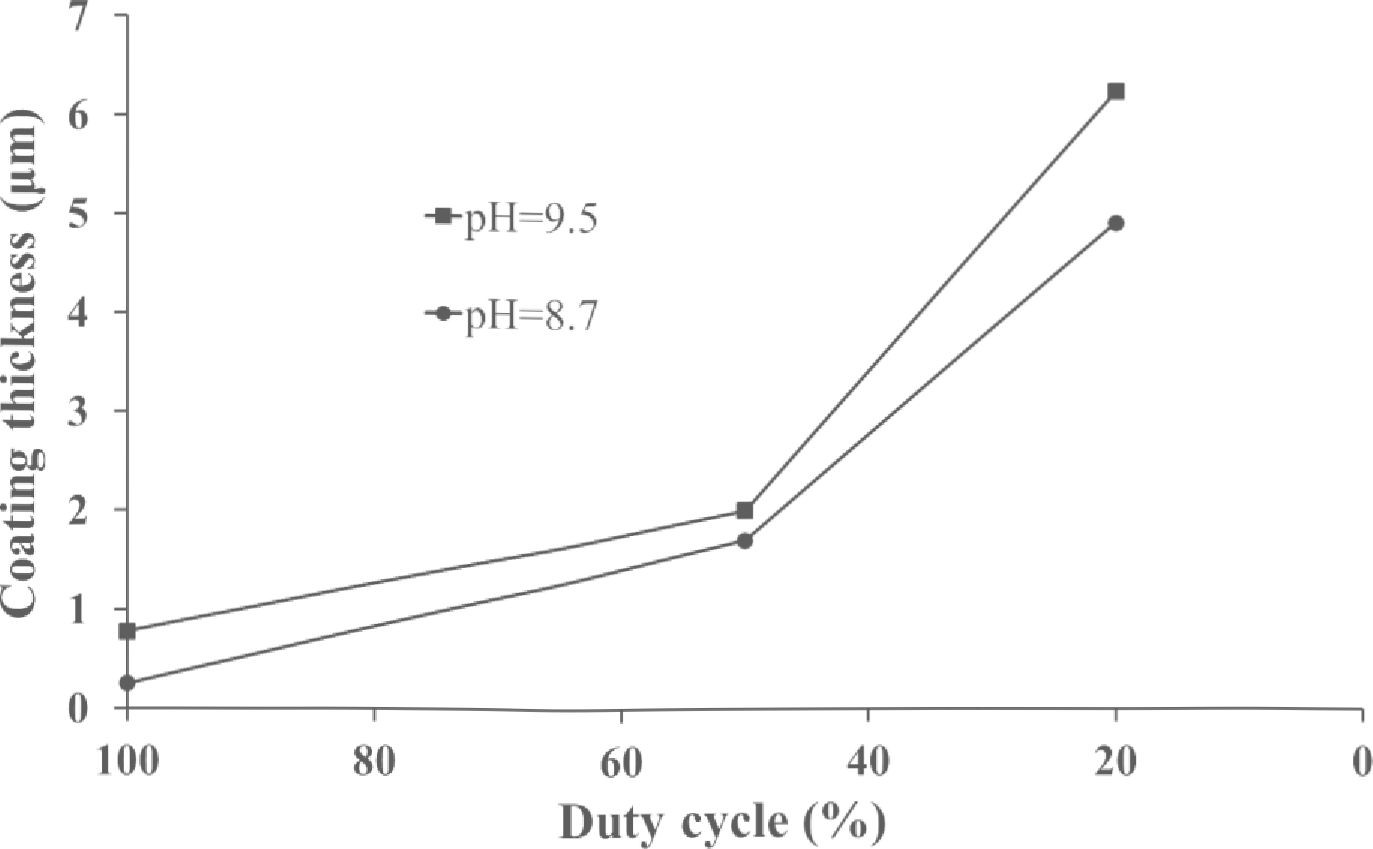
Fig. 7. The maximum thickness of crack - free coatings versus the duty cycle (the duty cycle of 100% corresponds to DC mode). The current density was 0.05 A.cm 2
The conducted studies have shown that the crack - free coatings of binary Ni-W alloy with a thickness more than 6.2 pm can be deposited using pyrophosphate electrolyte in the pulse current mode with duty cycle of up to 20 %. It was found that under the experimental conditions determined in this work, it is possible to fabricate high - quality coatings with rather homogenous and fine - crystalline structure (with the crystallites dimensions of 8 - 10 nm) without oxides, nitrides and intermetallic inclusions.
The following conclusions and recommendations on electrochemical deposition of binary Ni-W alloy using pyrophosphate electrolyte are formulated in order:
- The greatest Faradaic efficiency is reached for electrolyte with maximum stable pH value of 9.5 In that case, the optimal duty cycle is 20 %.
- The greatest W - content is reached for electrolyte with lower pH value of 8.7. At that, the dependences of W - content on the current density and duty cycle are rather weak.
- The surface morphology of coatings fabricated in electrolyte with pH 9.5 exhibits developed globular structure. An increase in the current density from 0.01 A.cm-2 to 0.05 A.cirT2 leads to an increase in the dimensions of individual globules in the range from 0.3 - 1.5 pm to 1 - 3 pm, which is accompanied by a certain decrease in their number.
Literature:
- Yamasaki T, «High - strength nanocrystalline Ni-W alloys produced by elcctrodeposition.» Mater. Phys. Mesh. 1 127- 132 (2000).
- Eliaz N. Sridhar TM, Gileadi E, «Synthesis and characterization of nickel tungsten alloys by electrodeposilion.» Electrochim. Acta 50 2893 - 2904 (2005).
- Sriraman KR, Ganesh Sundara Raman S. Seshadri SK. «Corrosion behavior of electrodeposited nanocrystalline Ni-W and Ni-Fe-W alloys.»Mater. Sei. Eng. A 460 39 - 45 (2007).
- Jones A, Hamaim J. Lund A, Schuh C, «Nanocrystalline Ni-W alloy coaling for engineering applications.» Plat. Surf. Finish. 97 52 - 60 (2010).
- Wasekar NP, Sundararajan G. «Sliding wear behavior of electrodeposited Ni-W alloy and Iiard chrome coalings» Wear 342 - 343 340 - 348 (2015).
- Brooman E. «Corrosion performance of environmentally acceptable alternatives to cadmium and cliromium coatings: Cliromium - Part II» Met Finish. 98 39 - 45 (2000).
- Kirihara S, Umeda Y, Tashiro K. Honma H, Takai O, «Development of Ni - W alloy plating as a substitution of hard chromium plating» Trans. Mater. Res. Soc. Jpn. 41 35-39 (2016).
- He F - j, Wang M, Lu X, «Properties of electrodeposited amorphous Fe-Ni-W alloy deposits.» Trans. Nonferrous Metals Soc. China 16 1289 - 1294 (2006).
- Allahyarzadeh MH, Aliofkhazraei M. Rezvanian AR, Torabinejad V, Sabour Rouhaghdam AR, «Ni - W electrodeposited coatings: Characterization, properties and applications.» Surf. Coat. Technol. 307 978- 1010(2016).
- Lee HB. «Synergy between corrosion and wear of electrodeposited Ni-W coating» Tribol. Lett. 50 407-419 (2013).
- Udompanit N, Wangyao P, Henpraserttae S, Boonyongmaneerat Y, «Wear response of composition - modulated InultilayerNi - W coatings» Adv. Mater. Res. 1025 - 1026 302 - 309 (2014).
- Amadeh A. Harsij sani M, Moradi H, «Wear behavior of carbon steel electrodeposited by IianociystalhneNi-W coatings.»//?/. J. ISSI6 14 - 19 (2009).
- Hosseini M. Abdolmaleki M, Ebralumzadesh H, Seyed Sadjadi S, «Effect of 2 - butyne - 1, 4 - diol on the nanostructure and corrosion resistance properties of electrodeposited Ni-W-B coatings» Int J. Electrochem. Sei. 6 1189 - 1205 (2011).
- Obradovic M. Stevanovic J, Despic A, Stevanoyic R, Stoch J, «Characterization and corrosion properties of electrodeposited Ni-W alloys» J. Serb. Chem. Soc. 66 899 - 912 (2001).
- Chianpairot A. Lothongkum G, Schuh CA, Boonyongmaneerat Y, «Corrosion of nanocrystalline Ni - W alloys in alkaline and acidic 3.5 wt.%NaCl solutions» Corros. Sei. 53 1066 - 1071 (2011).
- Alimadadi H, Alunadi M, Aliofkhazraei M, Younesi SR, «Corrosion properties of electrodeposited nanocrystalline and amorphous patterned Ni-W alloy»Mater. Des. 30 1356 - 1361 (2009).
- Yang Y, Zhang Y. Zliang Y, Yan B, Mo F, «Corrosion properties of ultrasonic electrodeposited nanocrystalline and amorphous patterned Ni-W alloy coatings» Mori. Phys. Lett. B 27 1341007 - 1 - 1341007-7 (2013).
- [18] Sassi W, Dhouibi L, Bcrcot P, Rezrazi M, Triki E, «Effect of pyridine on the electro - crystallization and corrosion behavior of Ni - W alloy coated from citrate - ammonia media» Appl. Surf. Sei. 263 373 -381 (2012).
- Yang FZ. Guo YF, Huang L, Xu SK, Zhou SM, «Electrodeposition, structure and corrosion resistance of nanocrystalline Ni-W alloy» Chin. J. Chem. 22 228 - 231 (2004).
- Metikos - Hukovic M, Grubac Z, Radic N, Tonejc A, «Sputter deposited nanocrystalline Ni and Ni - W films as catalysts for hydrogen evolution» J. Mol. Catal. A Chem. 249 172 - 180 (2006).
- Hristova E, Mitov M, Rashkov R, Amaudova M, Popov A, «Sulphide oxidation on electrodeposited Ni - Mo - W catalysts» Bulg. Chem. Commun. 40 291 - 294 (2008).
- Kawashima A, Akiyama E, Habazaki H, Hashimoto K, «Characterization of sputter deposited Ni - Mo and Ni-W alloy electrocatalysts for hydrogen evolution in alkaline solution» Mater. Sei. Eng. A 226 905-909 (1997).
- [23] Do TK, Lund А, «А reliability study of a new nano cry Stalline nickel alloy barrier layer for electrical contacts» Proc. 56th IEEE Holm Conf, on Electric Contacts, Charleston, USA, Oct. 2010, 73-81.
- Mizushima I, Tang PT, Hansen HN, Somers MA, «Residual stress in Ni - W electrodeposits» Electrochim. Acta 51 6128-6134 (2006).
- Wang M, Wang Z. Guo Z, «The Stracture evolution and stability of Ni - W films electrodeposited under super gravity field» Mater. Lett. 64 1166 - 1168 (2010).
- Zemanova M, Krivosudska M. Chovancova M. Jonk V. «Pulse current electrodeposition and corrosion properties of Ni - W alloy coatings» J. Appl. Electrochem. 41 1077 - 1085 (2011).
- Vasko AT, Electrochemistry of molybdenum and tungsten. Naukova Dumka. Kiev. 1977 (in Russian).
- Krasikov AV, Krasikov VL. «Mechanism of nickel - tungsten alloy electrodeposition from pyrophospliate electrolyte» Izvestiya SPbGTI 36 12 - 23 (2016) (in Russian).
- Suvorov DV, Gololobov GP. Tolstoguzov AB. Karabanov SM, Tarabrin DY, Rybin NB, Stnuchkova YM, Serpova MA. Korotchenko VA. "Electrodeposition of Ni - W gradient coatings.” J. Phys.: Conf Ser. 857 012047 (2017).
- Zhu L, Younes O, Ashkenasy N. Sliacham - Diamand Y. Gileadi E, «STM/AFM studies of the evolution of morphology of electroplated Ni/W alloys». Ippl. Surf. Sci. 200 1 - 14 (2002).
- N.P. Lyakishev, Diagrams of the state of double metal systems. Mashinoslroenie, Moscow, 2001, p. 872 (in Russian).